|
FMEA Analysis
Fathom can help you establish risk mitigation protocols and evaluation through a failure modes and effects analysis.
IRB Management
The rules and regulations of institutional review boards vary amongst pre-clinical trial sites. Let Fathom manage the animal testing facilities by fulfilling all of the necessary paperwork required by the institution prior to clinical evaluation.
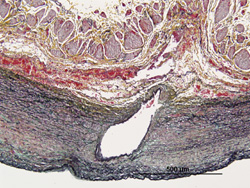
Biocompatibility
Depending upon the target location and length of exposure, a demonstration of biocompatibility (carcinogenicity, toxicity, etc.) may be required. Fathom can develop testing protocols and manage our partner labs to evaluate the biocompatibility of your products.
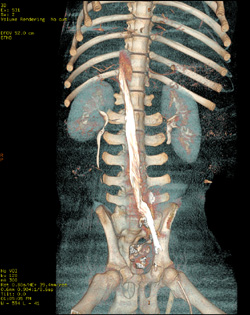
GLP Animal Studies
Identification of the appropriate animal model and the establishment of protocols for executing a complete and accurate GLP study can be conducted by Fathomís team of experts.
|
|







